|
|
Bronchopulmonary Sequestration
Submitted by Samir Jethra, MSIV
Bronchopulmonary Sequestration
- Bronchopulmonary sequestration (BPS) is a rare congenital malformation of the
lower respiratory tract.
- It consists of a nonfunctioning mass of normal lung tissue that lacks normal
communication with the tracheobronchial tree, and that receives its arterial blood
supply from the systemic circulation.
- BPS is estimated to comprise 0.15 to 6.4 percent of all congenital
pulmonary malformations, making it an extremely rare disorder.
- Sequestrations are classified anatomically.
- Intralobar sequestration (ILS) in which the lesion is located within a
normal lobe and lacks its own visceral pleura.
- Extralobar sequestration (ELS) in which the mass is located outside the
normal lung and has its own visceral pleura
- The blood supply of 75% of pulmonary sequestrations is derived from
the thoracic or abdominal aorta.
- The remaining 25% of sequestrations receive their blood flow from
the subclavian, intercostal, pulmonary, pericardiophrenic, innominate,
internal mammary, celiac, splenic, or renal arteries.
Intralobar sequestration
- The intralobar variety accounts for 75 percent of all sequestrations.
- Usually presents in adolescence or adulthood as recurrent pneumonias.
- Lies within the same visceral pleura as the lobe in which it occurs.
- Males and females are equally affected with ILS.
- In ILS, the arterial supply usually is derived from the lower thoracic or
upper abdominal aorta.
- Venous drainage is usually to the left atrium via pulmonary veins establishing
a left to right shunt.
- Abnormal connections to the vena cava, azygos vein, or right atrium may occur.
- Two thirds of the time, the sequestration is located in the paravertebral
gutter in the posterior segment of the left lower lobe.
- Unlike extralobar sequestration, it is rarely associated with other
developmental abnormalities.
- Patients present with signs and symptoms of pulmonary infection of a lower lobe mass.
- It is believed that sequestrations become infected when bacteria
migrate through the pores of Kohn or if the sequestration is incomplete.
Extralobar sequestration
- The extralobar variety accounts for 25 percent of all sequestrations.
- ELS usually presents in infancy with respiratory compromise.
- Develops as an accessory lung contained within its own pleura.
- ELS has a male predominance (80%).
- Related to the left hemidiaphragm in 90% of cases.
- ELS may present as a subdiaphragmatic or retroperitoneal mass.
- In general, the arterial supply of ELS comes from an aberrant vessel arising from the thoracic aorta.
- It usually drains via the systemic venous system to the right atrium, vena cava, or azygos systems.
- Congenital anomalies occur more frequently in patients with ELS than ILS.
- Associated anomalies include Congenital cystic adenomatoid malformation (CCAM), congenital diaphragmatic hernia, vertebral anomalies, congenital
heart disease, pulmonary hypoplasia, and colonic duplication
- Since it is enveloped in its own pleural sac, it rarely gets infected so
almost always presents as a homogeneous soft tissue mass.
- The mass may be closely associated with the esophagus, and fistulae may develop.
Imaging
- An arteriogram has been considered vital in documenting the systemic
blood supply, allowing definitive diagnosis as well as preoperative planning.
- The advent of new noninvasive imaging techniques has changed this thinking.
CHEST RADIOGRAPH
- Sequestrations typically appear as a uniformly dense mass within the thoracic
cavity or pulmonary parenchyma.
- Recurrent infection can lead to the development of cystic areas within the mass.
- Air-fluid levels due to bronchial communication can be seen.
ULTRASOUND
- The typical sonographic appearance of BPS is an echogenic homogeneous
mass that may be well defined or irregular.
- Some lesions have a cystic or more complex appearance.
- Doppler studies are helpful to identify the characteristic aberrant systemic
artery that arises from the aorta and to delineate venous drainage.
CT
MRI
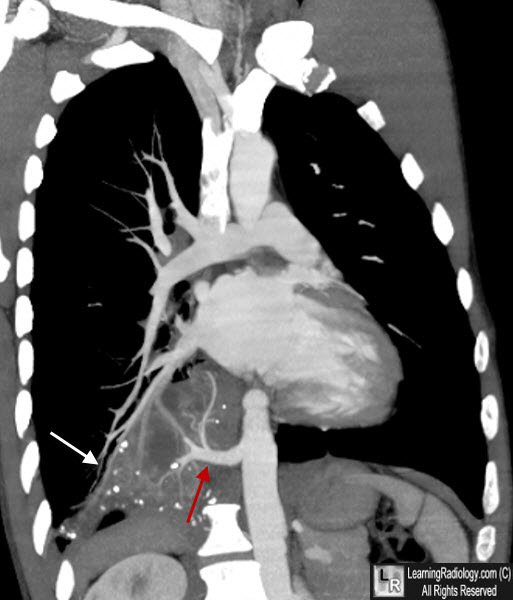
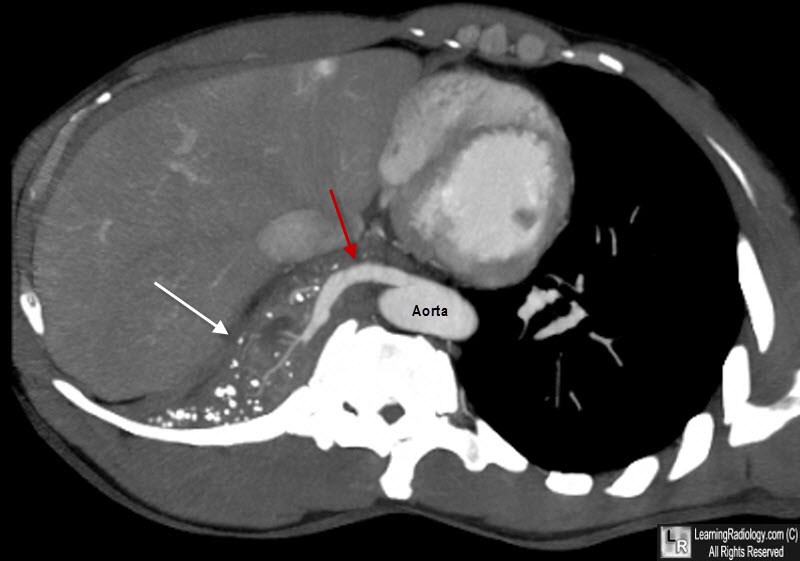
Intralobar Sequestration, Right Lower Lobe. There is a mass in the right lower lobe (white arrows) which is drawing its blood supply (red arrows) from the descending aorta. Drainage was back to the pulmonary veins.
Grainger & Allison's Diagnostic Radiology: A Textbook of Medical Imaging, 4th ed., 2001 Churchill Livingstone, Inc. pp 654-655.
Khan, Ali Nawaz, Bronchopulmonary Sequestriation, e-Medicine, http://www.emedicine.com/radio/topic585.htm.
Oermann, Christopher M, Bronchopulmonary Sequestration, Up to Date, http://www.utdol.com/application/topic.asp?file=pedipulm/10425&type=P&selectedTitle=4~6
|
|
|